Overview
The regulation of health products relies on standards, guidelines, and legal frameworks. Effective governance, ensuring timely access to safe, high-quality, and effective medicines for patients, depends on collaboration among stakeholders including the industry, regulatory authorities, healthcare professionals, and patients.
This foundational course explores the roles and contributions of various stakeholders, key functions, and guidelines that shape the regulatory landscape. It provides comprehensive insights into the end-to-end processes of pharmaceutical development, addressing regulatory requirements throughout the product lifecycle and contemporary regulatory approaches.
Course Description & Learning Outcomes
Explain the foundational basis of regulatory management and decision-making processes for pharmaceutical products, highlighting the key concepts involved in managing pharmaceuticals across their lifecycles
Understand the regulatory requirements for the different product development phases and learn about real-world regulatory decision-making through interactive, practical sessions
Learn about the key regulatory organisations driving innovation of regulatory processes and policies

Schedule
End Date: 19 Sep 2025, Friday
Monday - Friday, 830 AM - 530 PM
Location: Duke-NUS Medical School, 8 College Rd, Singapore 169857, 169857Pricing
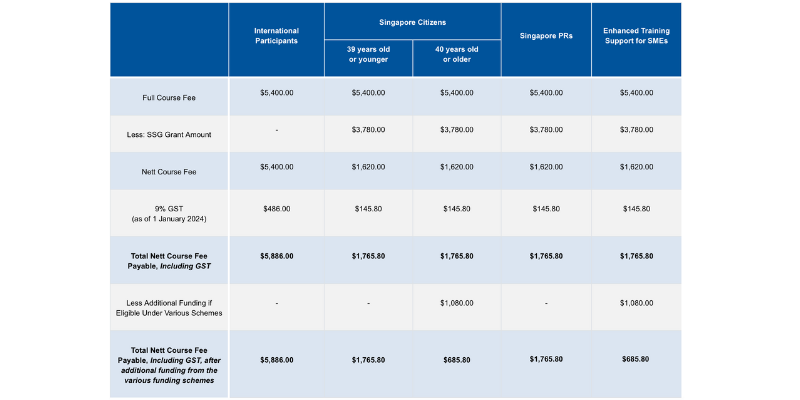
Course fees: SGD
Skills Covered
PROFICIENCY LEVEL GUIDE
Beginner: Introduce the subject matter without the need to have any prerequisites.
Proficient: Requires learners to have prior knowledge of the subject.
Expert: Involves advanced and more complex understanding of the subject.
- Product Management (Proficiency level: Beginner)
- Product Development (Proficiency level: Beginner)