Overview
One of the key factors for ensuring and maintaining the safety and efficacy of medical technology is the regulatory control of manufacturing, which is supported by a robust Quality Management System (QMS). A well-implemented QMS is pivotal to successful medical technology design and development, ensuring compliance with international standards, regulatory controls and audits, as well as maintaining product quality post-market entry.
This course covers the production processes, quality management systems, and regulatory activities related to quality assurance of medical technology in regulated markets.
Course Description & Learning Outcomes
Explain the fundamentals of Good Manufacturing Practices for medical technology
Articulate the concepts and basis of Quality Management Systems in relation to regulatory requirements (in particular the ISO 13485)
Describe key quality management processes for raw materials, sites, and facilities in manufacturing of medical devices

Schedule
End Date: 21 Nov 2025, Friday
Monday - Friday, 830 AM - 530 PM
Location: Academia, SGH Campus, 20 College Rd, Singapore 169856, 169856Pricing
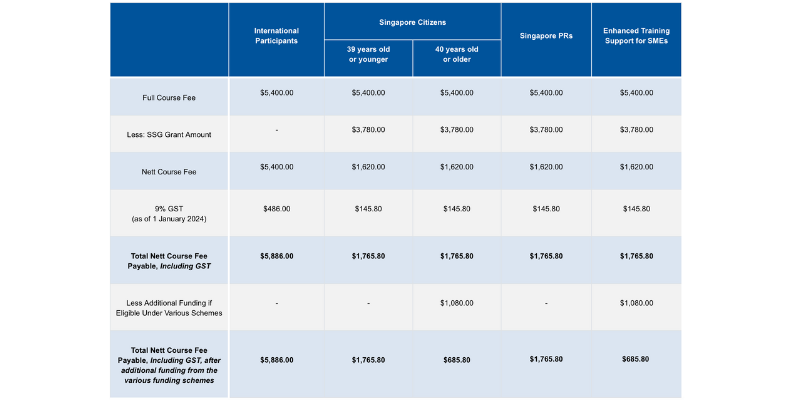
Course fees: SGD
Skills Covered
PROFICIENCY LEVEL GUIDE
Beginner: Introduce the subject matter without the need to have any prerequisites.
Proficient: Requires learners to have prior knowledge of the subject.
Expert: Involves advanced and more complex understanding of the subject.
- Product Development (Proficiency level: Beginner)
- Quality assurance (QA) (Proficiency level: Beginner)