Overview
In today's digital world, technologies and software play an increasingly important role in healthcare management- diagnosis, treatment, patient monitoring and real-world data collection. Software-based medical devices require more streamlined and efficient regulatory oversight due to their higher turnaround times, need for continuous validation and verification, as well as robust software development practices. Effective governance should account for the dynamic nature of software development, ensuring timely compliance and continuous improvement to maintain safety, quality, and efficacy.
Software standards and guidelines published by standards development organisations (e.g., ISO, IEC) and regulatory agencies/forums are important tools to aid effective governance, allowing timely access for patients. This course covers essential topics including regulatory frameworks, risk management principles, and post-market surveillance for digital health products.
Course Description & Learning Outcomes
List and describe the relevant standards and guidances required in Digital Health products verification and validation
Describe regulatory activities through medical device software development, testing and documentation
Describe key regulatory considerations in the product life cycle including change management and post-market activities for digital health products

Schedule
End Date: 30 May 2025, Friday
Monday - Friday, 830 AM - 530 PM
Location: Duke-NUS Medical School, 8 College Rd, Singapore 169857, 169857Pricing
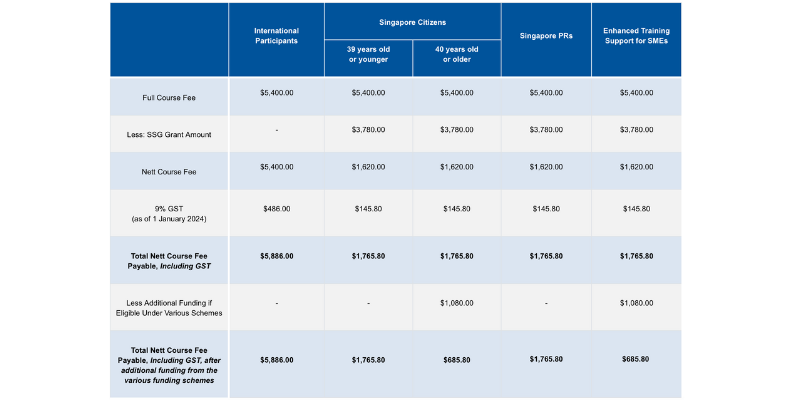
Course fees: SGD
Skills Covered
PROFICIENCY LEVEL GUIDE
Beginner: Introduce the subject matter without the need to have any prerequisites.
Proficient: Requires learners to have prior knowledge of the subject.
Expert: Involves advanced and more complex understanding of the subject.
- Product Development (Proficiency level: Beginner)
- Product Management (Proficiency level: Beginner)






