Overview
The assurance of safe and quality medicines in the market depends on a range of vital activities after the approval of a medicine by the authorities. This includes the continual monitoring offered by inspections and audits of facilities and testing of product quality. In this globalised environment, there is also an increasing need to leverage on networks to effectively detect lapses in product quality and services in a timely manner.
This course introduces the key activities and roles essential for effective post-market control, including management of failures of conformance and compliance. Topics covered will include collaborations and networks for optimising post-market communications, and handling of substandard and falsified pharmaceuticals.
Course Description & Learning Outcomes
Understand the damaging implications of poor quality medicines to public health, as well as the evolving challenges in minimising the entry of these substandard medicines and falsified products.
Explain the regulatory controls along the entire distribution and supply chain of medicines, and the good practices and standards expected.
Gain practical insights on the assessment of quality defects and detection of falsified medicines.

Schedule
End Date: 09 May 2025, Friday
Monday - Friday, 830 AM - 530 PM
Location: Duke-NUS Medical School, 8 College Rd, Singapore 169857, 169857Pricing
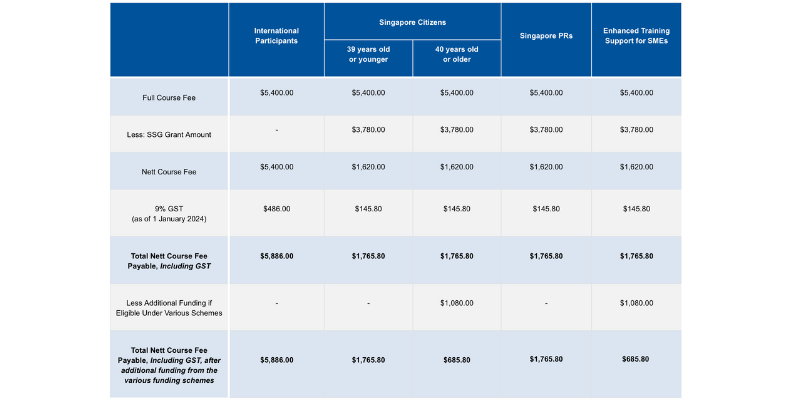
Course fees: SGD
Skills Covered
PROFICIENCY LEVEL GUIDE
Beginner: Introduce the subject matter without the need to have any prerequisites.
Proficient: Requires learners to have prior knowledge of the subject.
Expert: Involves advanced and more complex understanding of the subject.
- Product Development (Proficiency level: Beginner)
- Product Management (Proficiency level: Beginner)