Overview
This 5-day, in-person course provides the foundation in understanding established standards, guidelines and regulatory principles on conformity assessment of medical device softwares.
Course Description & Learning Outcomes
In today’s digital world, technologies and software play an increasingly important role in healthcare management - diagnosis, treatment, patient monitoring and Real World Data collection. While software-based medical devices products are currently controlled via medical device regulation, they differ significantly from traditional medical devices requiring a more streamlined and efficient regulatory oversight. Software standards and guidelines published by standards development organisations (eg., ISO, IEC) and regulatory agencies/forums are important tools for effective governance for safe, quality and efficacious health products and timely access for patients. At the end of this course, you will be able to: - List and describe the relevant standards and guidances required in Digital Health products verification and validation - Describe regulatory activities through medical device software development, testing and documentation - Describe key regulatory considerations in the product life cycle including change management and post-market activities
Recommended Prerequisites
The Admissions Committee reviews all application material, reference letters, and interviewer comments carefully to better understand each applicant. Applicants must have an undergraduate Bachelor’s degree or its equivalent in the relevant discipline. Relevant work experience (i.e., in the fields of regulatory, healthcare or research) at the time of matriculation into Duke-NUS Medical School is desirable. Applicants from non-English speaking countries will be required to present documentary evidence of their competence in written and spoken English (TOEFL or ILS).
Pre-course instructions
Online Preparatory Materials will be emailed to participants 1 month prior to the workshop. Programme will be uploaded to CoRE’s website approximately 2 weeks before the workshop date. For participants who are supported by SSG, please note that if you have taken an SSG-funded course, you would be liable to pay back the SSG training grant plus the prevailing Goods and Services Tax (GST) to NUS if you have received the grant for the course and fall into any of the following categories: - Failure to fulfil a minimum attendance of 75% for the course - Premature withdrawal from the course - Failure to pass all prescribed coursework, examinations and/or assessments for the course - Please note that the Tuition Fee and Student Services Fee (SSF) are non-refundable and non-transferable Registration close date: 28/07/2023
Schedule
Date: 30 Oct 2023, Monday
Time: 8:30 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: Academia, 20 College Rd, 169856
Date: 31 Oct 2023, Tuesday
Time: 8:30 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: Academia, 20 College Rd, 169856
Date: 01 Nov 2023, Wednesday
Time: 8:30 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: Academia, 20 College Rd, 169856
Date: 02 Nov 2023, Thursday
Time: 8:30 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: Academia, 20 College Rd, 169856
Date: 03 Nov 2023, Friday
Time: 8:30 AM - 5:30 PM (GMT +8:00) Kuala Lumpur, Singapore
Location: Academia, 20 College Rd, 169856
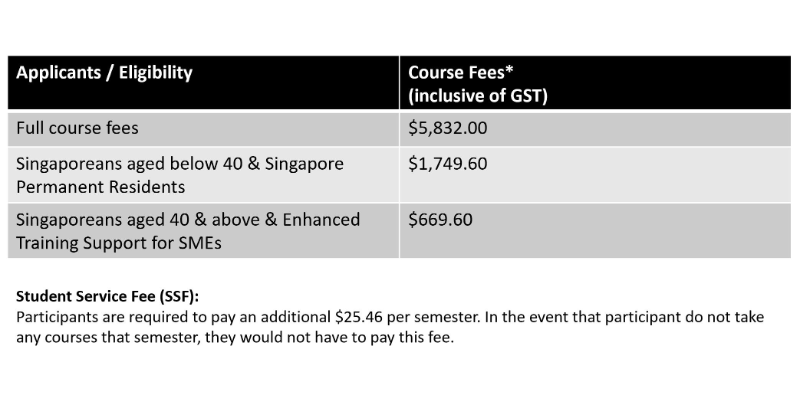
Pricing